Abstract
Lignin is considered an ideal natural material for the production of sustainable monophenols. In this study, a microwave-assisted depolymerization (MAD) strategy was developed. The introduction of solvent vapors in the dynamic vapor flow reaction system was performed to enhance the lignin conversion efficiency. The results showed that no liquid products were generated from the MAD of lignin without solvent vapors. With the introduction of solvents (CH3OH, HCHO, HCOOH, and CH2Cl2), liquid products appeared (especially with CH2Cl2, which had the highest yield of 41.9 wt%). Results from gas chromatography/mass spectrometry of liquid products showed that seven kinds of compounds, including guaiacols, phenols, syringols, methoxyphenyls, heterocycles, esters, and aromatics were identified. CH2Cl2 can significantly enhance the production of monophenols (guaiacols, phenols, and syringols). The introduction of these solvent vapors can also facilitate the generation of porous char with high Brunauer-Emmett-Teller specific surface areas. Some carbon nanospheres deposited on the surface of the char were obtained with the assistance of CH2Cl2. This study provides a facile method for the utilization of lignin in the field of bio-based fine chemicals.
Keywords
With the depletion of fossil resources and the threat of global warming, the global community has focused on exploring renewable sources instead of fossil-based fuels and chemicals
Pyrolysis is one of the most promising technologies for the conversion of lignin into renewable chemicals, such as monophenols. As an important and effective pyrolysis method, microwave-assisted depolymerization (MAD) has attracted extensive attention owing to its ultra-fast heating, flexible control (no thermal inertia), high thermal efficiency, and special selectivity in chemical reactions
Based on the above discussion, a new strategy involving the introduction of solvent vapors as the carrier gas for the MAD system was developed for the lignin depolymerization process. This dynamic vapor flow reaction system was designed to draw support from solvent vapors to reform the intermediates (pyrolysis vapor) and restrain the lignin fragment condensation, finally obtaining the target products of monophenols from lignin. In a previous study on the cleavage of 5-5′ C (phenyl)—C (phenyl) bonds in the kraft lignin model
Lignin (CAS: 9005-53-2) was purchased from TCI (Shanghai) Development Co., Ltd. Methanol (CH3OH, AR), formaldehyde (HCHO, AR), formic acid (HCOOH, AR), dichloromethane (CH2Cl2, AR), and silicon carbide (SiC, CAS: 409-21-2) were purchased from Aladdin Industrial Corp.
The experiments were performed in a self-developed MAD reactor equipped with a device for solvent-vapor input. SiC particles are good microwave absorbents for the rapid heating of lignin materials (lignin has poor microwave absorptivity). In this study, 10 g of lignin mixed well with 20 g of SiC particles was placed in the MAD reactor. N2 (600 mL/min) flowed continuously through the reactor for 10 min to remove air prior to experiments. Subsequently, different solvents (CH3OH, HCHO, HCOOH, CH2Cl2) were added to a gas-washing bottle. N2 flowed through the gas-washing bottle and then went to the reactor. The microwave reactor was operated with a constant microwave power of 1000 W (2.45 GHz). The reactor was maintained for 15 min after reaching 600℃ . The volatile vapor produced by depolymerization was cooled by rapid condensation at (-35±1)℃ with a cooling medium of ethanol and collected as liquid products. The solid products were obtained in the MAD reactor and collected after cooling.
The chemical composition of the liquid products was determined by gas chromatography/mass spectrometry (GC-MS, 6892N/5975I, Agilent). The liquid samples obtained at 600℃ with different solvents (CH3OH, HCHO, HCOOH, and CH2Cl2) were automatically injected into the GC/MS for analysis. The peaks were identified using the NIST11 library. The conditions for GC-MS were as follows: inlet temperature of 280℃, He gas flow rate of 1.0 mL/min, and split-flow ratio of 30∶1. The heating schedule was preset such that the temperature remained at 50℃ for 5 min, increased to 280℃ at a rate of 5℃/min, and then was maintained for 7 min. A junction temperature of 280℃ , ion temperature of 230℃ , electron ionization source electron energy of 70 eV, and scanning range of 18-500 μm were used for MS.
The functional groups of the liquid products were characterized by a Fourier transform infrared (FT-IR) analyzer (VERTEX 70, Bruker). Hydrogen nuclear magnetic resonance
The Brunauer-Emmett-Teller (BET) specific surface area and pore structures of the solid products were evaluated by N2 adsorption-desorption isotherms at 77 K using a Micromeritics ASAP2460 analyzer. The surface morphology was investigated using field emission scanning electron microscopy (FE-SEM, S-4800, Hitachi).
The experiments were performed in the MAD reactor using a dynamic vapor flow reaction system (
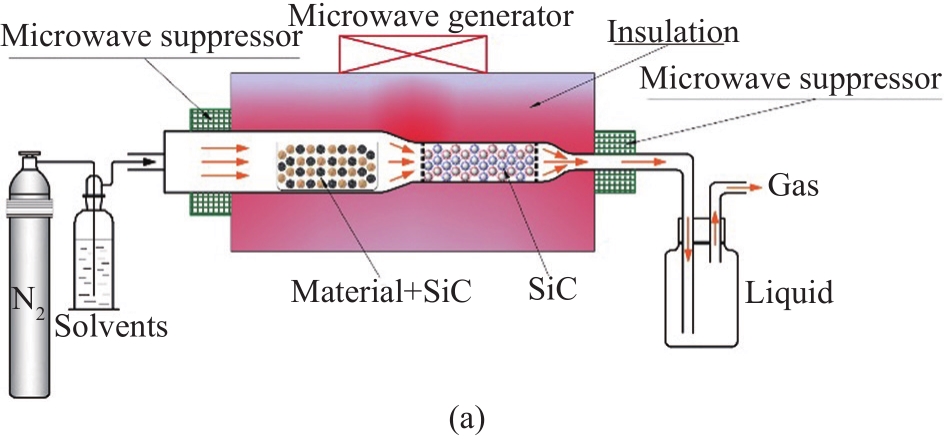
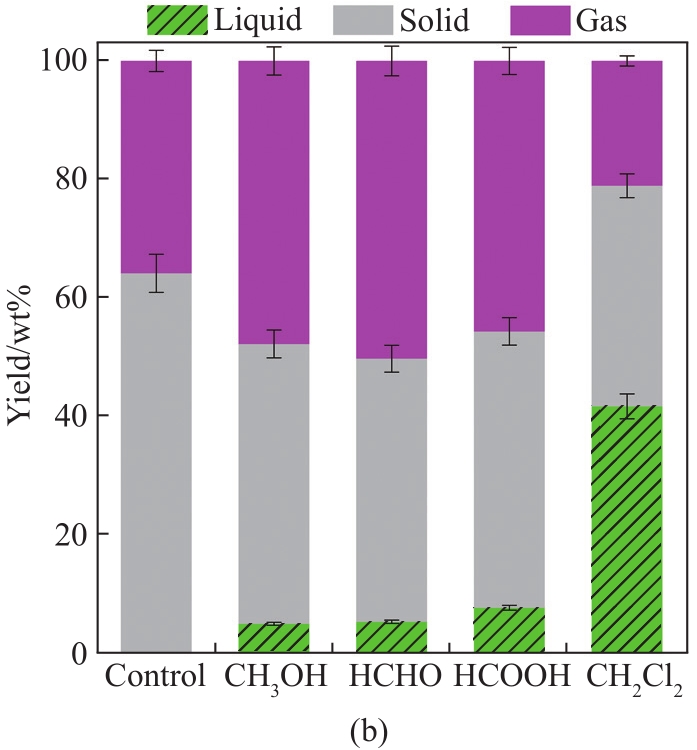
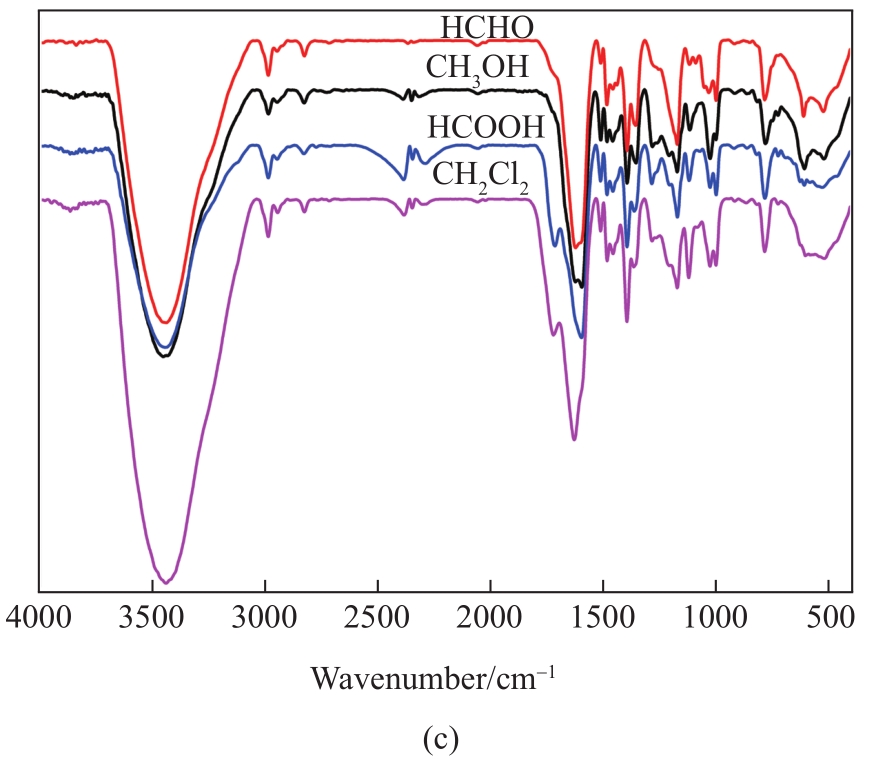
Fig. 1 (a) Process for MAD of lignin. (b) Yields of the liquid, gas, and solid products, and (c) FT-IR spectra of liquid products using different solvents
As shown in
FT-IR (
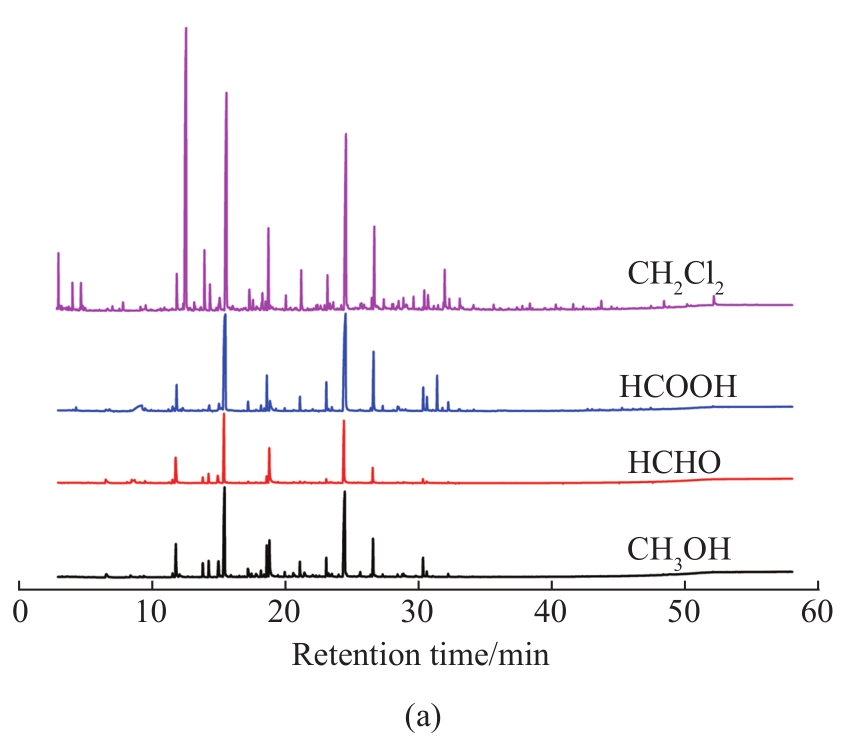
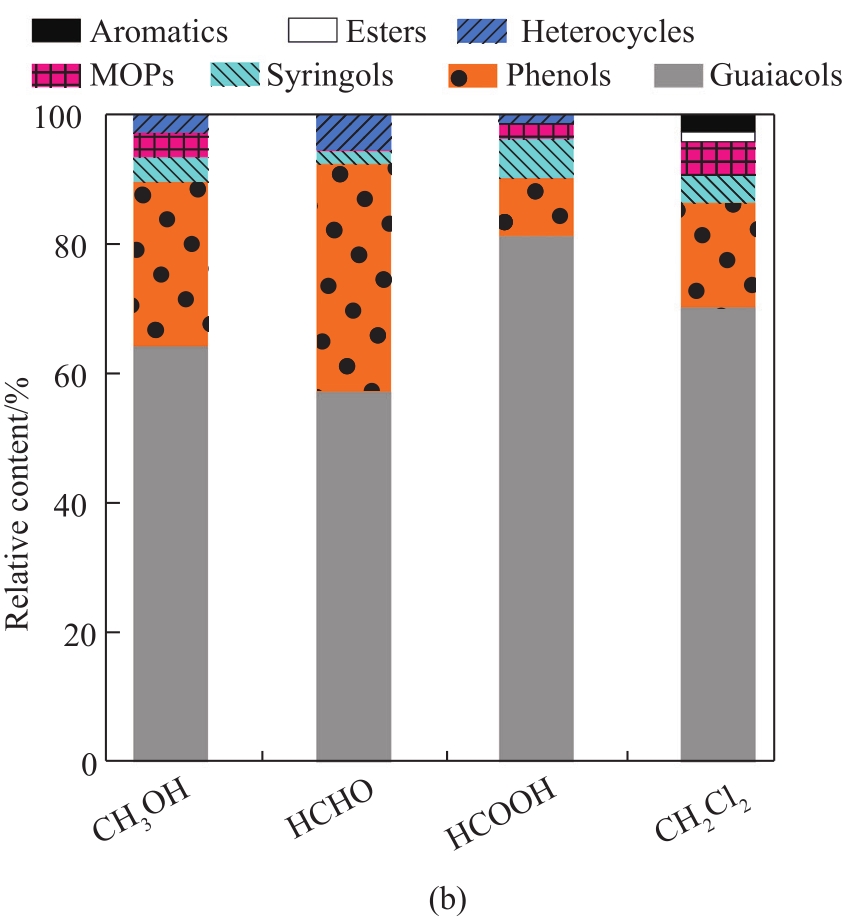
Fig. 2 (a) Total ion chromatograms of liquid products from MAD of lignin using different solvents; (b) component distribution of liquid products
To further understand the role of these solvents during lignin depolymerization under microwave irradiation, the chemical composition of the liquid products was investigated by GC-MS (
To further analyze the component distribution quantitatively, peak areas were used to reflect the content variation of the corresponding compounds. As shown in
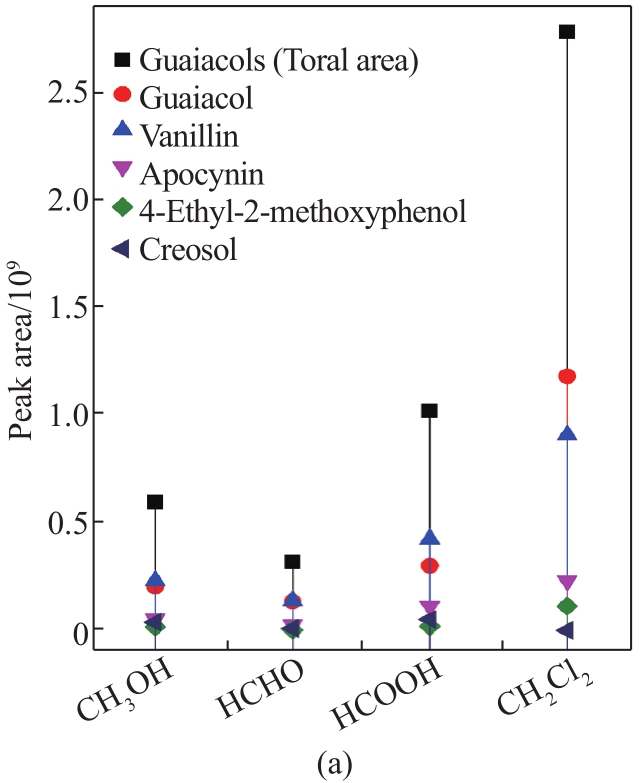
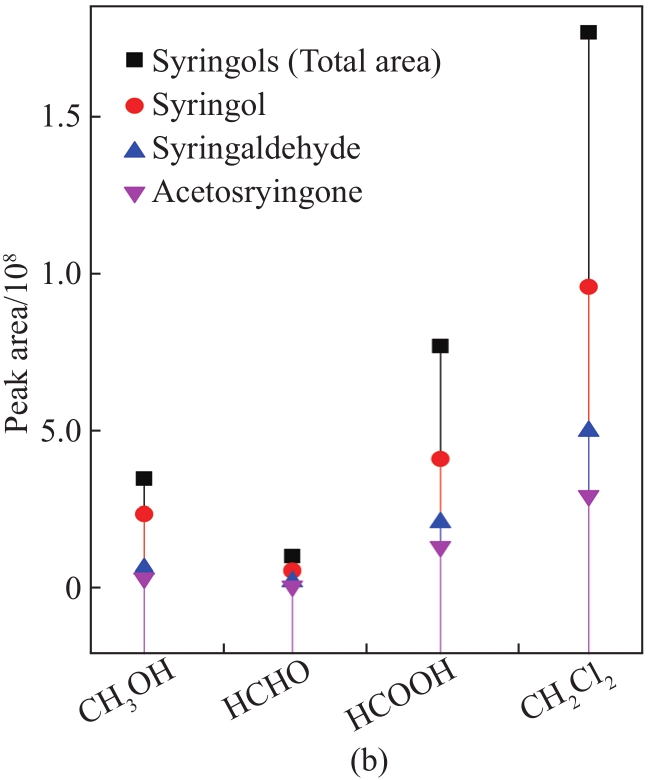
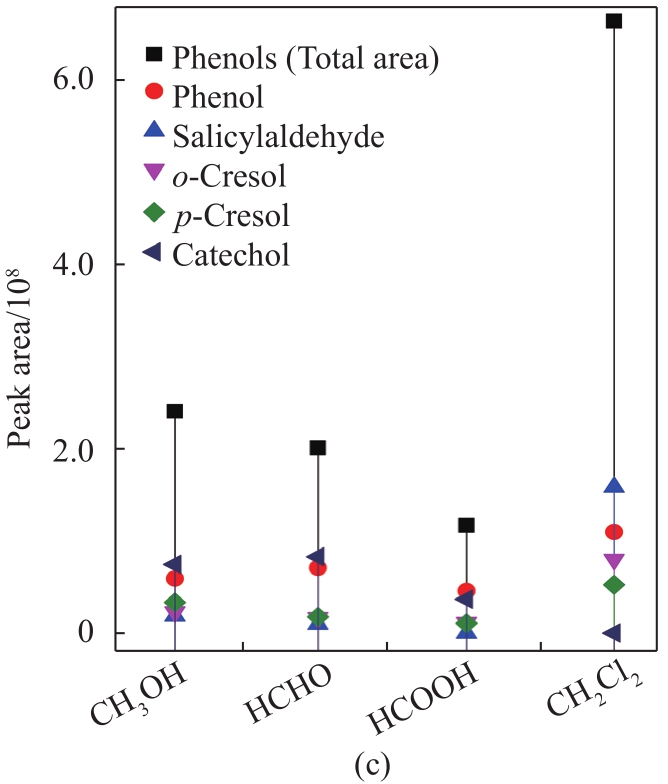
Fig. 3 Peak area of guaiacols (a), syringols (b), and phenols (c) in liquid products from MAD of lignin using different solvents
Overall, the contents of guaiacols, phenols, and syringols with CH2Cl2 were much higher than those with CH3OH, HCHO, and HCOOH, respectively, indicating the enhanced effect of the introduction of lignin depolymerization with CH2Cl2. In this case, CH2Cl2 vapor may provide a strong reductive environment by the generation of hydrogen radicals from the electromagnetic field effect of microwave irradiation
The structural characteristics of the liquid products were determined by
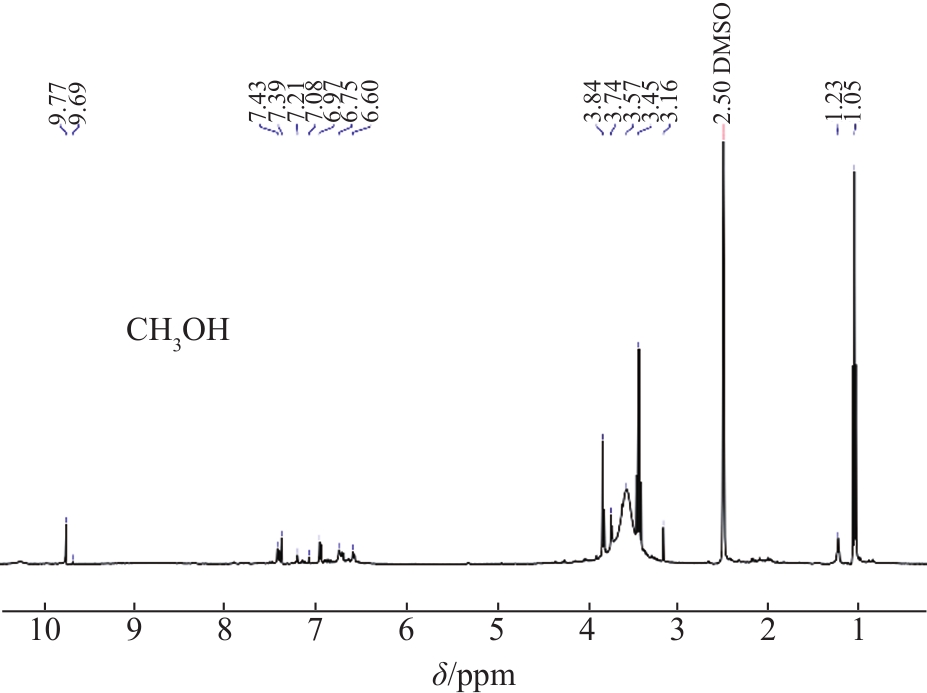
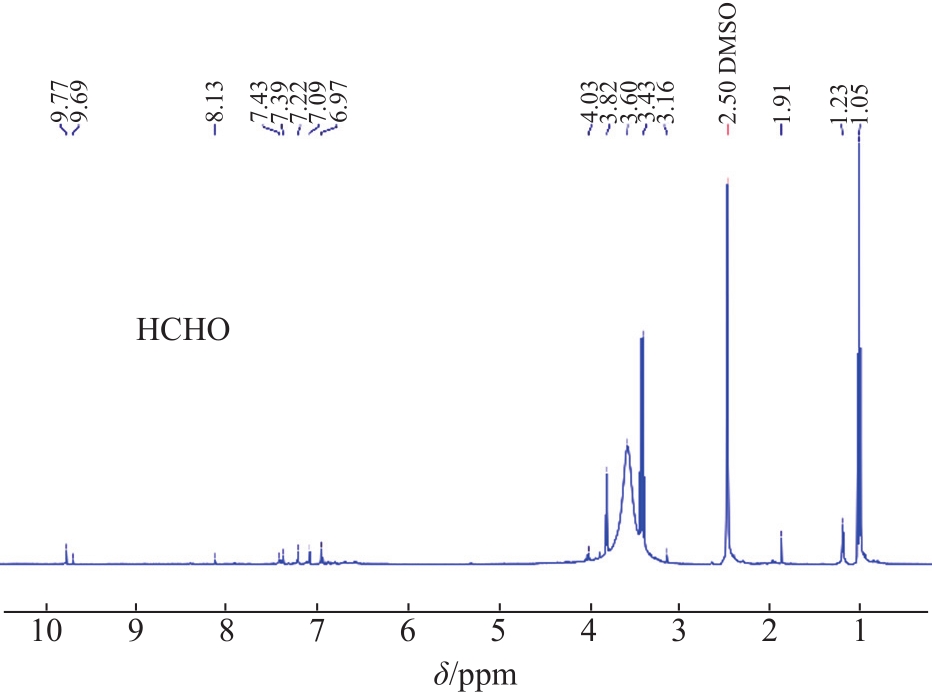
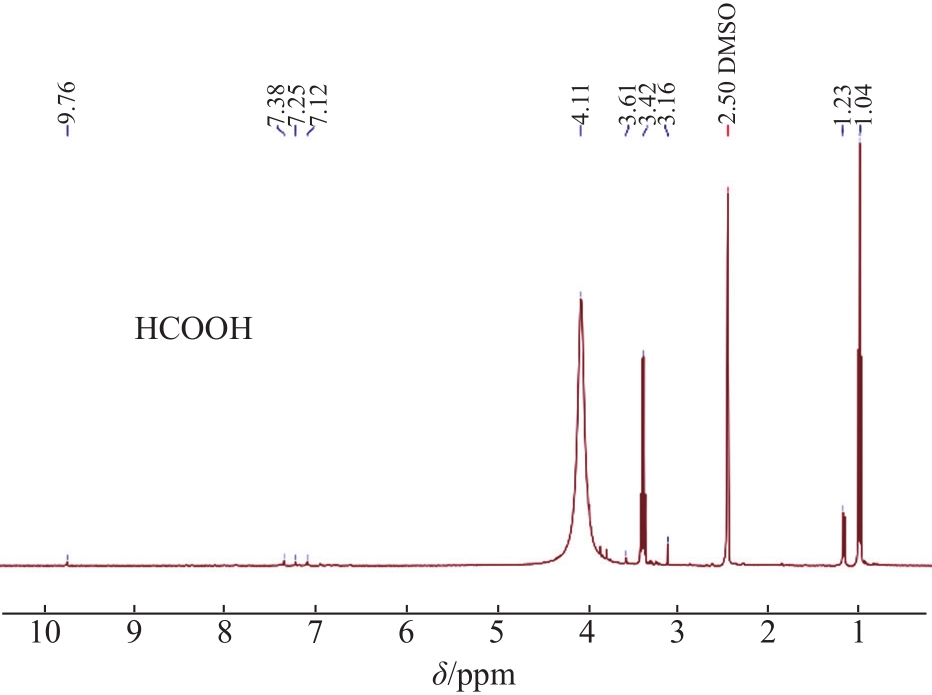
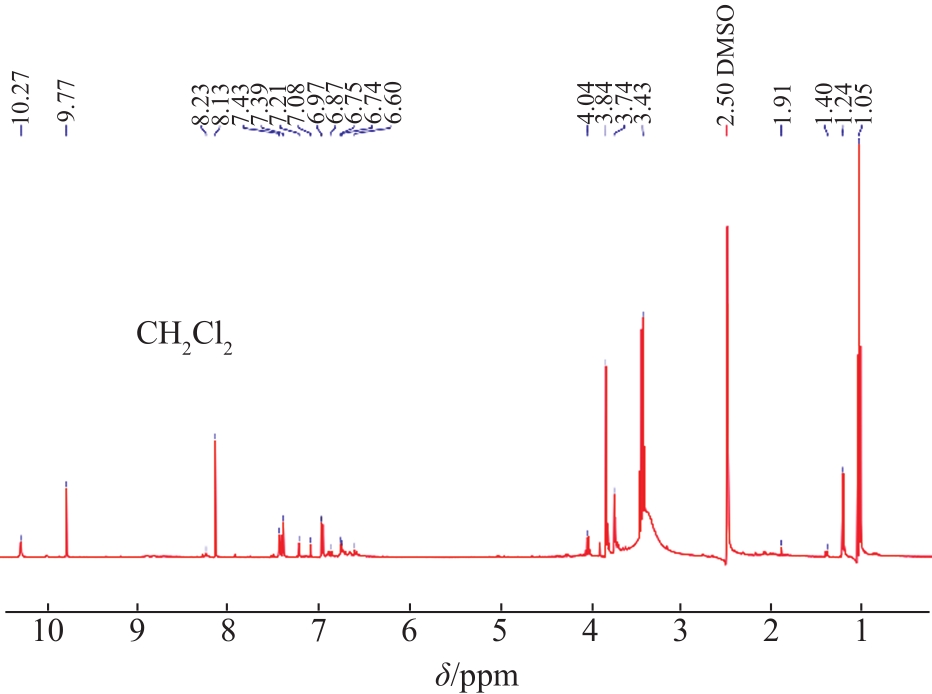
Fig. 4
Solid products are usually produced from the intramolecular and intermolecular rearrangement of intermediates during the lignin decomposition process with the formation of an aromatic polycyclic struc-ture

Fig. 5 (a-f) FE-SEM images of solid products from MAD of lignin using different solvents. (a) Lignin material; (b) control; (c) CH3OH; (d) HCHO; (e) HCOOH; (f) CH2Cl2; (g) N2 adsorption-desorption isotherms of solid products
The solid char from the MAD of lignin with CH2Cl2 presented significantly different shapes, with some carbon nanospheres inlayed on the surface of the char (
Based on the microwave-assisted dynamic vapor flow reaction system, solvent vapors were injected to successfully depolymerize lignin into renewable monophenols. The introduction of solvents (CH3OH, HCHO, HCOOH, and CH2Cl2) facilitated the production of liquid products. Seven kinds of compound such as guaiacols, phenols, syringols, methoxyphenyls, heterocycles, esters, and aromatics were identified in the liquid products. The solvent vapor of CH2Cl2 significantly enhanced the conversion efficiency of lignin for monophenols (guaiacols, syringols, and phenols). In addition, the introduction of these solvents can facilitate the generation of porous char with high Brunauer-Emmett-Teller specific surface areas. This work demonstrated that solvent vapor (especially CH2Cl2) has a positive effect on the production of monophenols. The results provide an efficient and facile approach to enhance the value of lignin as an alternative to petroleum for fine chemicals.
Acknowledgements
This work was supported by the Foundation of the Key Laboratory of Pulp and Paper Science and Technology of the Ministry of Education of China (No. KF201917) and the National Natural Science Foundation of China (31800497).
References
Huang K F, Fasahati P, Maravelias C T. System-level Analysis of Lignin Valorization in Lignocellulosic Biorefineries. Iscience, 2020, DOI:10.1016/j.isci.2019.100751. [Baidu Scholar]
Zakzeski J, Bruijnincx P C A, Jongerius A L, Weckhuysen B M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chemical Reviews, 2010, 110 (6), 3552-3599. [Baidu Scholar]
Wang W L, Li X P, Ye D, Cai L P, Sheldon Q. Catalytic pyrolysis of larch sawdust for phenol-rich bio-oil using different catalysts. Renewable Energy, 2018, 121, 146-152. [Baidu Scholar]
Liu C, Wu S, Zhang H, Xiao R. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review. Fuel Processing Technology, 2019, 191, 181-201. [Baidu Scholar]
Zhang C F, Wang F. Catalytic Lignin Depolymerization to Aromatic Chemicals. Accounts of Chemical Research, 2020, 53(2), 470-484. [Baidu Scholar]
Hu J J, Zhang Q G, Lee D J. Kraft lignin biorefinery: A perspective. Bioresource Technology, 2018, 247, 1181-1183. [Baidu Scholar]
Qin X Y, Duan C, Feng X M, Zhang Y L, Dai L, Xu Y J, Ni Y H. Integrating phosphotungstic acid-assisted prerefining with cellulase treatment for enhancing the reactivity of kraft-based dissolving pulp. Bioresource Technology, 2021, DOI: 10.1016/j.biortech.ww.124283. [Baidu Scholar]
Zhang X S, Rajagopalan K, Lei H W, Ruan R, Sharma B K. An overview of a novel concept in biomass pyrolysis: microwave irradiation. Sustainable Energy & Fuels, 2017, 1 (8), 1664-1699. [Baidu Scholar]
Zhang Y N, Cui Y L, Liu S Y, Fan L L, Zhou N, Peng P, Wang Y P, Guo F Q, Min M, Cheng Y L, et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresource Technology, 2020, 297, 122480-122488. [Baidu Scholar]
Beneroso D, Monti T, Kostas E T, Robinson J. Microwave pyrolysis of biomass for bio-oil production: Scalable processing concepts. Chemical Engineering Journal, 2017, 316, 481-498. [Baidu Scholar]
Wang H L, Pu Y Q, Ragauskas A, Yang B. From lignin to valuable products-strategies, challenges, and prospects. Bioresource Technology, 2019, 271, 449-461. [Baidu Scholar]
Shen X J, Meng Q L, Mei Q Q, Liu H Z, Yan J, Song J L, Tan D X, Chen B F, Zhang Z R, Yang G Y, et al. Selective catalytic transformation of lignin with guaiacol as the only liquid product. Chemical Science, 2020, 11(5), 1347-1352. [Baidu Scholar]
Yu X N, Wei Z Q, Lu Z X, Pei H S, Wang H L. Activation of lignin by selective oxidation: An emerging strategy for boosting lignin depolymerization to aromatics. Bioresource Technology, 2019, DOI: 10.1016/j.biortech.2019.121885. [Baidu Scholar]
Zhang X H, Ma H, Wu S B. Effects of temperature and atmosphere on the formation of oligomers during the pyrolysis of lignin. Fuel, 2020, DOI: 10.1016/j.fuel.2020.117328. [Baidu Scholar]
Wu Q H, Wang Y P, Jiang L, Yang Q, Ke L Y, Peng Y J, Yang S, Dai L L, Liu Y H, Ruan R. Microwave-assisted catalytic upgrading of co-pyrolysis vapor using HZSM-5 and MCM-41 for bio-oil production: Co-feeding of soapstock and straw in a downdraft reactor. Bioresource Technology, 2020, 299, 122611-122618. [Baidu Scholar]
Wang W L, Wang M, Huang J L, Tang N, Dang Z P, Shi Y J, Zhaohe M H. Microwave-assisted catalytic pyrolysis of cellulose for phenol-rich bio-oil production. Journal of the Energy Institute, 2019, 92(6), 1997-2003. [Baidu Scholar]
Zhang S, Jiang S F, Huang B C, Shen X C, Chen W J, Zhou T P, Cheng H Y, Cheng B H, Wu C Z, Li W W, et al. Sustainable production of value-added carbon nanomaterials from biomass pyrolysis. Nature Sustainability, 2020, DOI: 10.1038/s41893-020-0538-1.1. [Baidu Scholar]
Wang W L, Wang M, Li X P, Cai L P, Shi S Q, Duan C, Ni Y H. Microwave-Assisted Catalytic Cleavage of C-C Bond in Lignin Models by Bifunctional Pt/CDC-SiC. ACS Sustainable Chemistry & Engineering, 2020, 8(1), 38-43. [Baidu Scholar]
Luo X L, Li Y D, Gupta N K, Sels B, Ralph J, Shuai L. Protection Strategies Enable Selective Conversion of Biomass. Angewandte Chemie-International Edition, 2020, 59(29), 11704-11716. [Baidu Scholar]
Adam M, Ocone R, Mohammad J, Berruti F, Briens C. Kinetic Investigations of Kraft Lignin Pyrolysis. Industrial & Engineering Chemistry Research, 2013, 52(26), 8645-8654. [Baidu Scholar]
Asawaworarit P, Daorattanachai P, Laosiripojana W, Sakdaronnarong C, Shotipruk A, Laosiripojana N. Catalytic depolymerization of organosolv lignin from bagasse by carbonaceous solid acids derived from hydrothermal of lignocellulosic compounds. Chemical Engineering Journal, 2019, 356, 461-471. [Baidu Scholar]
Raikwar D, Majumdar S, Shee D. Thermocatalytic depolymerization of kraft lignin to guaiacols using HZSM-5 in alkaline water-THF co-solvent: a realistic approach. Green Chemistry, 2019, 21(14), 3864-3881. [Baidu Scholar]
Shu R Y, Xu Y, Ma L L, Zhang Q, Wang C, Chen Y. Controllable production of guaiacols and phenols from lignin depolymerization using Pd/C catalyst cooperated with metal chloride. Chemical Engineering Journal, 2018, 338, 457-464. [Baidu Scholar]
Wang W L, Wang X B, Ma Z H, Duan C, Liu S W, Yu H L, Li X P, Cai L P, Shi S Q, Ni Y H. Breaking the lignin conversion bottleneck for multiple products: Co-production of aryl monomers and carbon nanospheres using one-step catalyst-free depolymerization. Fuel, 2021, 285, 119211-119218. [Baidu Scholar]
Shuai L, Amiri M T, Questell-Santiago Y M, Heroguel F, Li Y D, Kim H, Meilan R, Chapple C, Ralph J, Luterbacher J S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science, 2016, 354(6310), 329-333. [Baidu Scholar]
Chen J N, Xu W T, Zhu J, Wang X Y, Zhou J C. Highly effective direct decomposition of H2S by microwave catalysis on core-shell Mo2N-MoC@SiO2 microwave catalyst. Applied Catalysis B-Environmental, 2020, DOI: 10.1016/j.apcatb.2019.118454. [Baidu Scholar]
Bhattacharya M, Basak T. A review on the susceptor assisted microwave processing of materials. Energy, 2016, 97, 306-338. [Baidu Scholar]
Paysepar H, Rao K T V, Yuan Z S, Shui H F, Xu C. Production of phenolic chemicals from hydrolysis lignin via catalytic fast pyrolysis. Journal of Analytical and Applied Pyrolysis, 2020, 149, 104842. [Baidu Scholar]
Ma H, Li T F, Wu S B, Zhang X H. Effect of the interaction of phenolic hydroxyl with the benzene rings on lignin pyrolysis. Bioresource Technology, 2020, DOI: 10.1016/j.biortech.2020.123351. [Baidu Scholar]
Wang X, Liu Y C, Chen M Z, Luo M, Yang P, Chen W M, Zhou X Y. Direct Microwave Conversion from Lignin to Micro/Meso/Macroporous Carbon for High-Performance Symmetric Supercapacitors. ChemElectroChem, 2019, 6(18), 4789-4800. [Baidu Scholar]
Zhong G, Xu S M, Cui M J, Dong Q, Wang X Z, Xia Q Q, Gao J L, Pei Y, Qiao Y, Pastel G, et al. High-Temperature, In Situ Microwave Synthesis of Bulk Nanocatalysts. Small, 2019, 15(47), 1904881-1904889. PBM [Baidu Scholar]