Abstract
Pretreatment is important for achieving high-value utilization of biomass. This study is conducted to evaluate the destruction of the Moso bamboo cell wall via hydrothermal pretreatment at different temperatures and pH values. Compositional and morphological analyses and subsequent enzymatic hydrolysis of the solid fractions indicate that the destruction of the cell wall, instead of the degradation or removal of hemicellulose and lignin, or the configuration transition of the cellulose crystal structure, is the most critical aspect for improving bioconversion efficiency. Although only an 8%-10% weight loss is incurred and similar crystalline indexes are achieved after mild hydrothermal treatments, the recovery of glucose is doubled, whereas the recovery of xylose from pretreated samples is approximately 35%.
Biomass is defined as all living organic matter that can be grown, including all plants, microorganisms, and animals, and is an important resource of renewable energy. The efficient development and utilization of biomass energy will facilitate the alleviation of the energy crisis and ecological environmental problems. The gradual increase in global energy consumption, fossil fuel depletion, and concerns regarding climate change have necessitated the development of sustainable energy systems based on renewable resources
resource, is suitable for the production of bio-based materials, including biofuels and chemicals. The plant cell wall primarily comprises cellulose, hemicellulose, and lignin, which are tightly linked through covalent, intermolecular, and intramolecular hydrogen bonds to form a complex three-dimensional structure that prevents biotransformation degradation. Pretreatment processes are extensively employed to release the cellulose component embedded in the heterogeneous matrix of the plant cell wall, which exhibits a high contact surface and hydrophilicity
Bamboo is an important sustainable and renewable non-timber forest resource in China; it offers the advantages of abundant reserves, fast growth, high strength and hardness, superior processing performance, and local availability. Bamboo is a promising perennial plant that is distributed worldwide; it exhibits specific growth characteristics and features required for the production of natural materials. Bamboo fibers exhibit good physicochemical and mechanical properties; hence, they are a potential alternative to conventional materials used in polymer composites
In recent years, researchers have investigated the mechanisms of various pretreatment techniques to enable the biodegradation of the cell wall; in this regard, significant progress has been achieved. Hydrothermal treatment with or without a catalyst, as a low-cost and environmentally friendly process, can partially separate hemicellulose and lignin from cellulose, disrupt the intact structure of lignocellulose, and improve the enzymatic bioconversion efficiency of cellulosic substrates. In this study, the complexity and diversity of the bamboo cell wall, as well as the corresponding variation during mild hydrothermal pretreatment (with or without acidic or alkaline catalyst) and bioconversion were emphasized. It is envisioned that the dissociation of non-cellulosic components and ultrastructural changes in different morphological regions will be established, thereby providing a theoretical foundation for the fractionation mechanism of bamboo fibers.
Moso bamboo was harvested in Anhui Province; subsequently, it was airdried and crushed into powder. Between 40 and 60 meshes were allocated for chemical analysis and pretreatment. The bamboo was cut into small pieces (1 cm × 1 cm) and sliced to a thickness of 20 μm using a slicer (Yamato REM710, Japan) after being softened in a 90℃ water bath for approximately 20 d. All samples were stored in a 4℃ refrigerator, and all chemicals used in this study were of analytical grade and used directly.
The powder samples were fractionated in a sealed stainless-steel autoclave (volume 50 mL) lined with polytetrafluoroethylene (PTFE). Five grams of bamboo powder or 20 pieces of bamboo slices were mixed with deionized water, 0.1 wt% H2SO4, and 0.1 wt% NaOH (labeled as P, PH, and POH), respectively, at a solid-liquid ratio of 1∶10, where the raw material was completely immersed in the liquid. After tightening the reactor, the mixture was heated to the desired temperature (100℃, 120℃, and 140℃) and maintained for 3 h. Subsequ⁃ently, the reactor was removed promptly from the oven and cooled gradually to room temperature. Finally, the solid cellulosic fractions were accumulated via filtration, washed thoroughly with distilled water until a neutral pH value was achieved, and then freeze dried. The detailed parameters in addition to the corresponding designations of the samples are listed in
The composition of structural carbohydrates in the samples was determined based on the National Renewable Energy Laboratory Protocol and analyzed via high-performance liquid chromatography (HPLC, Waters e2695, USA) on an Aminex HPX-87H analytical column (300 mm × 7.8 mm, Bio-Rad Laboratories, USA). The analysis involved a two-step acid hydrolysis to determine the carbohydrate content, a weighing process to measure the content of acid-insoluble lignin (AIL, i.e., Klason lignin), and a spectral process to determine the content of acid-soluble lignin (ASL). The powder samples were hydrolyzed with 72% H2SO4 (by weight) for 1 h at room temperature with intermittent stirring and then diluted with pure water to 4%, followed by a hydrothermal treatment at 105℃ for another 2 h. Finally, the hydrolysate was directly analyzed via HPLC after being filtered using filter paper. The solid residue was oven dried to a constant weight and then used to calculate the content of acid-insoluble lignin
| (1) |
where B is the lignin content in hydrolysis solution, g/L; A is the UV adsorption at 205 nm; D is dilution factor; 110 is the absorbance factor, L/(g·cm).
The Fourier transform infrared (FT-IR) spectra of the selected samples were recorded on an FT-IR spectrophotometer (Bruker Tensor II, Germany) using the KBr disc method at a ratio of 100∶1; subsequently, the samples were ground to 74 μm to form a flake. The spectra were recorded in the absorption mode in the 500-4000 c
| (2) |
where CrI is the relative crystallinity, I002 the diffraction intensity of the 002 plane, and Iam the diffraction intensity of the amorphous region.
Morphological changes in the cell wall of Moso bamboo before and after pretreatment and bioconversion were compared using optical and electronic techniques. After washing the bamboo slices thoroughly using deionized water, they were clamped onto slides using forceps and then shielded with coverslips to ensure the integrity of the slices before being observed under an optical microscope (Cewei LW300-28LT, China). After being airdried and sputtered with gold-palladium in a sputter coater (Hitachi SU8010, Japan), the original and pretreated samples were observed via scanning electron microscopy (SEM, S3400N, Hitachi, Japan) at an accelerating voltage of 5 kV.
The initial substrate (fractionated samples) concentration was set to 10% (w/V) in 30 mL of sodium acetate buffer (pH value 4.8) in a 100 mL conical flask, where 35 FPU/g of substrate loaded with enzyme (Novozymes Biotechnology Ltd., China) was introduced to a constant-temperature water-bath bed shaken at a rotating speed of 150 r/min at 30℃ for 72 h. The supernatant was sampled periodically for the following measurements after it was inactivated in a boiling water bath for 5 min
| (3) |
where BE is the bioconversion efficiency, MG the amount of Glc released; MC the amount of cellulose in substrate.
Fractionation efficiency is typically regarded as the primary target for the pretreatment process and entire utilization of bamboo. Three parallel experiments for each condition were performed, and the standard deviations obtained for all data were less than 5% (
By comparing the positions of the diffraction peaks in the XRD curves (
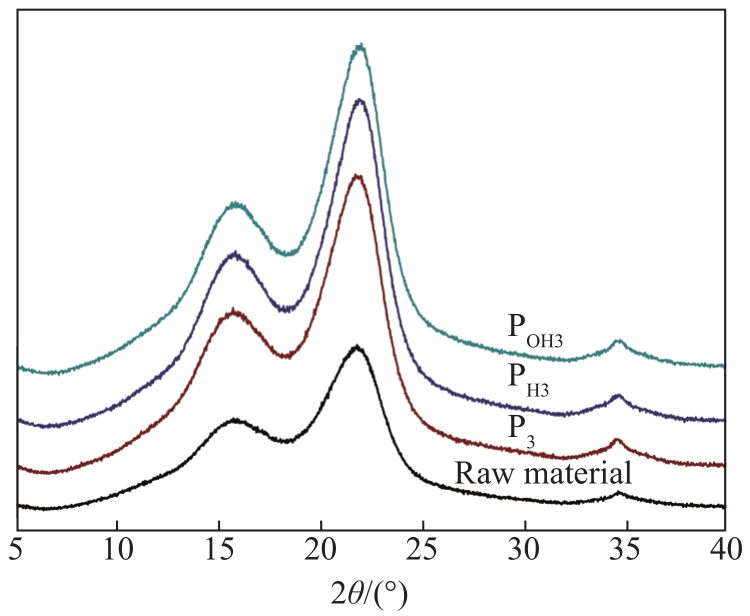
Fig. 1 X-ray diffractograms of raw material and fractionated samples under mild conditions
The variation in the functional groups in the fractionated samples was further examined using FT-IR technology (
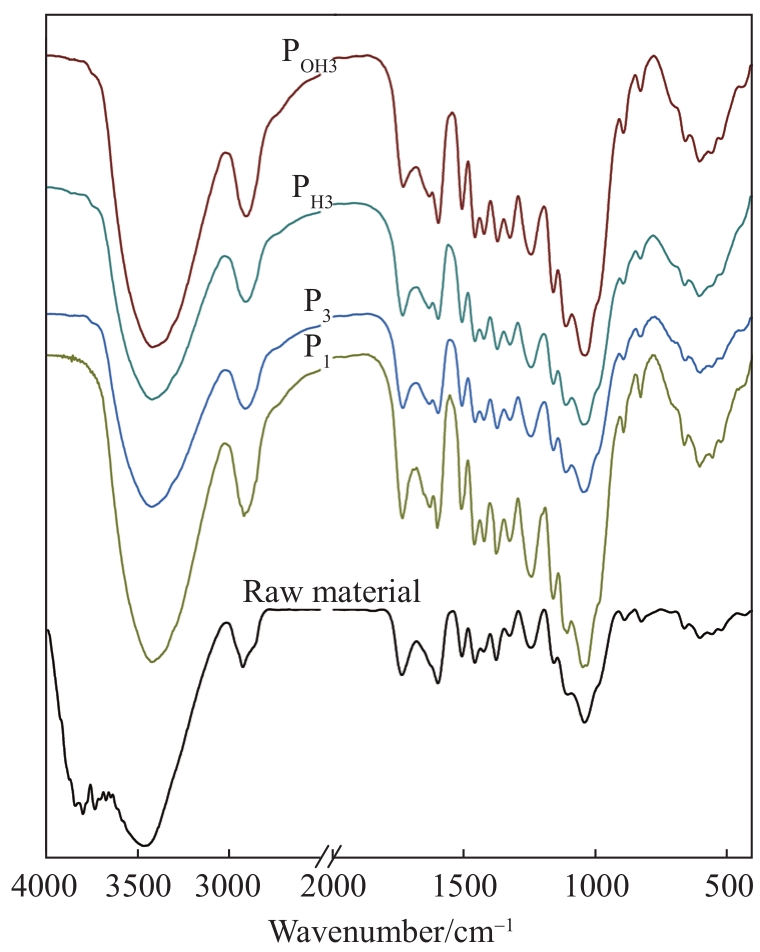
Fig. 2 FT-IR spectra of raw material and fractionated samples under mild conditions
The modification of physicochemical properties observed in the cellulosic substrates can affect the subsequent bioconversion process. As shown in
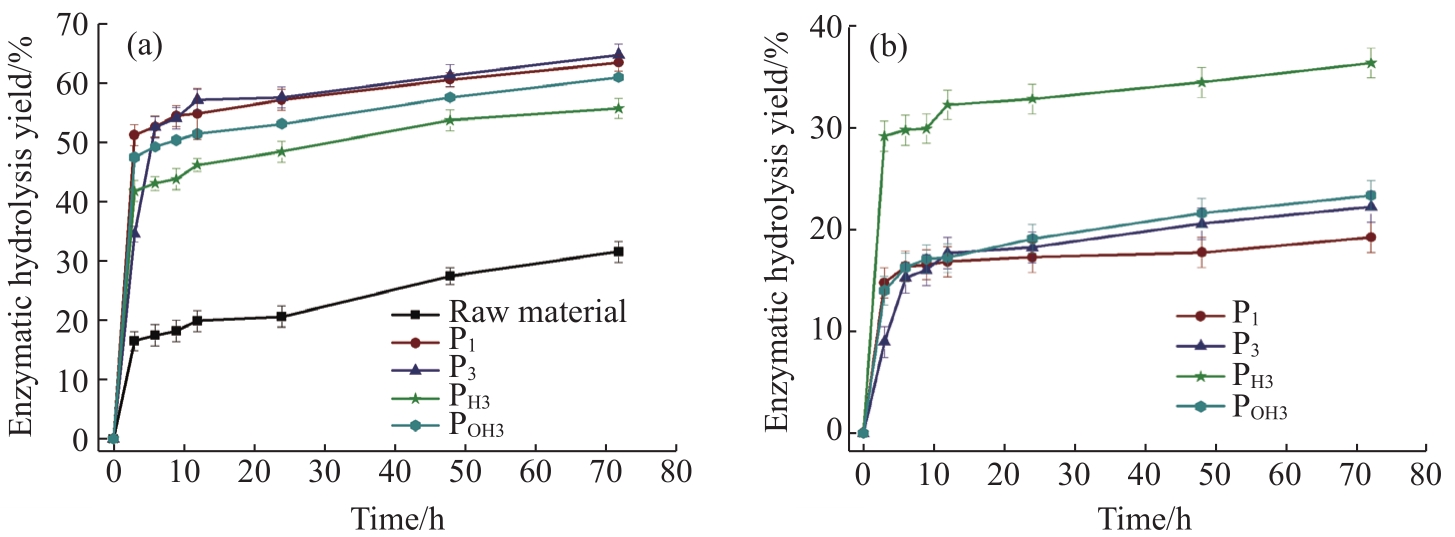
Fig. 3 Effect of pretreatment conditions on recovery of glucose (a) and xylose (b) from fractionated samples after enzymatic hydrolysis
As discussed above, the compositional analysis, cellulose crystal configuration, and functional groups did not change significantly during the mild pretreatment processes. Therefore, the significantly improved enzymatic efficiency was primarily ascribed to the morphological variation of the bamboo cell wall. The surface and cross-section of the raw and pretreated samples were recorded and compared (

Fig.4 Optical and SEM images of bamboo slices before (first and second columns) and after pretreatment (third column) and enzymatic biodegradation (fourth column)
The cross-sectional view shows that the variations in the cell wall and cell corner middle lamella were pronounced. The cell walls were intact and organized neatly; meanwhile, the cellulose and hemicellulose aggregated primarily in the primary and secondary walls, and lignin was assembled in the secondary walls. Clearly, the integrity and arrangement of the bamboo cell walls remained after hydrothermal treatment at 100℃. Increasing the temperature to 140℃ induced the softening and loosening of the stable structure, and further thinned the cell walls by the addition of acid. As mentioned above, the mild alkaline condition did not effectively degrade or remove the non-cellulosic matrix; however, the swelling of the cell walls was reflected in the images. The bamboo slices were more severely damaged by the enzyme compared with by the pretreatment, and some intervals and indicators of destruction were observed in the raw bamboo after biological degradation. Results pertaining to the recovery of monosaccharides are shown in
In this study, hydrothermal pretreatment with or without acidic or basic catalysts was performed to change the compact structure of the Moso bamboo cell wall considerably such that the bioconversion efficiency of bamboo can be increased significantly. The compositional, physical, and morphological characteristics, as well as the enzymatic digestibility of the solid fractions of Moso bamboo, were compared. The data indicated that the content of the main components and the crystal configuration of cellulose remained the same under mild hydrothermal conditions; however, the recovery of glucose improved significantly from 30.2% to 63.6%. A maximum of 35% hemicellulose (represented as xylose) was dissolved and detected in the hydrolysate. Based on the morphological variation of the cell walls, it was concluded that the destruction of compact cell walls was the most important aspect for increasing both the accessibility of cellulase to cellulose and the bioconversion efficiency for bioenergy applications.
Acknowledgements
This study was supported by a grant from the Natural Science Foundation of China (No. 31770622).
References
Agbor V B, Cicek N, Sparling R, Berlin A, Levin D B. Biomass pretreatment: fundamentals toward application. Biotechnology Advances: An International Review Journal, 2011, 29(6), 675-685. [Baidu Scholar]
Lynd L R, Weimer P J, Zyl W H, Pretorius I S. Microbial cellulose utilization: fundamentals and biotechnology. Microbio⁃logy and Molecular Biology Reviews, 2002, 66(3), 506-577. [Baidu Scholar]
Divya D, Indran S, Bharath K N. Bamboo: A Potential Natural Material for Bio-composites. Berlin: Springer, 2020. [Baidu Scholar]
Gao J, Qu L, Qian J, Wang Z, He Z. Effects of combined acid-alkali and heat treatment on the physiochemical structure of Moso bamboo. Scientific Reports, 2020, DOI: 10.1038/s41598-020-63907-7. [Baidu Scholar]
Wang K, Xu F, Sun R. Molecular characteristics of Kraft-AQ pulping lignin fractionated by sequential organic solvent extraction. International Journal of Molecular Sciences, 2010, 11(8), 2988-3001. [Baidu Scholar]
Lin J, Ndlovu L M, Singh S, Pillay B. Purification and biochemical characteristics of β -D-xylanase from a thermophilic fungus Thermomyces lanuginosus. Biotechnology & Applied Biochemistry, 1999, 30(1), 81-87. [Baidu Scholar]
Yamashita Y, Shono M, Sasaki C, Nakamura Y. Alkaline peroxide pretreatment for efficient enzymatic saccharification of bamboo. Carbohydrate Polymers, 2010, 79(4), 914-920. [Baidu Scholar]
Yang H, Chen Q, Wang K, Sun R C. Correlation between hemicelluloses-removal-induced hydrophilicity variation and the bioconversion efficiency of lignocelluloses. Bioresource Technology, 2013, 147, 539-544. [Baidu Scholar]
Qin L, Liu Z H, Li B Z, Dale B E, Yuan Y J. Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresource Technology, 2012, 112(1), 319-326. [Baidu Scholar]
Wang K, Yang H, Yao X, Feng X, Sun R C. Structural transformation of hemicelluloses and lignin from triploid poplar during acid-pretreatment based biorefinery process. Bioresource Technology, 2012, 116(4), 99-106. [Baidu Scholar]
Dong H L, Zhang A L, Lou R, Lou R, Xia C T. Effects of chemical pretreatment on the thermal-conversion properties of Moso bamboo. Paper and Paper Making, 2014, 33(11), 38-41. [Baidu Scholar]
Chen Q, Chen J, Wang K, Jiang J, Sun R. Research progress on the hydrothermal pretreatment of lignocellulosic biomass and its bioconversion. Scientia Silvae Sinicae, 2017, 53(9), 97-104. [Baidu Scholar]
Hayakawa K, Maruyama N, Fukasawa M. Capsule compri⁃sing low-substituted cellulose ether and method for preparing the same: US Patent, US20148784883 B2. 2014-07-22. [Baidu Scholar]
Gierlinger N, Goswami L, Schmidt M, Burgert I. In situ FT-IR microscopic study on enzymatic treatment of poplar wood cross-sections. Biomacromolecules, 2008, 9(8), 2194-2201. [Baidu Scholar]
Herrera R, Erdocia X, Llano-Ponte R, Labidi J. Characterization of hydrothermally treated wood in relation to changes on its chemical composition and physical properties. Journal of Analytical & Applied Pyrolysis, 2014, 107(5), 256-266. [Baidu Scholar]
Sun S, Sun S, Cao X, Sun R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresource Technology, 2016, 199, 49-58. [Baidu Scholar]
Tang S, Xu C, Vu L, Liu S, Ye P, Li L, Wu Y, Chen M, Xiao Y, Wu Y. Enhanced enzymatic hydrolysis of Pennisetum alopecuroides by dilute acid, alkaline and ferric chloride pretreatments. Molecules, 2019, DOI: 10.3390/molecules24091715. [Baidu Scholar]
Huang L, Yang Z, Li M, Liu Z, Yao S. Effect of pre-corrected pH on the carbohydrate hydrolysis of bamboo during hydrothermal pretreatment. Polymers, 2020, DOI: 10.3390/polym12030612. [Baidu Scholar]
Hao S, Sun Y, Zhang D, Huang C, Ma X. Effects of hydrothermal pretreatment of different corn stalks for fermentable sugar production. Journal of Hebei University of Technology, 2016, 45(3), 85-92. [Baidu Scholar]
Song X, Jiang Y, Rong X, Wei W, Wang S, Nie S. Surface characterization and chemical analysis of bamboo substrates pretreated by alkali hydrogen peroxide. Bioresource Technology, 2016, 216, 1098-1101. [Baidu Scholar]
Wang K, Feng X, Sun R, Gwynn J L. Influence of incubation time on the physicochemical properties of the isolated hemicelluloses from steam-exploded lespedeza stalks. Industrial & Engineering Chemistry Research, 2010, 49(18), 8797-8804. [Baidu Scholar]
Kristensen J B, Thygesen L G, Felby J E. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnology for Biofuels, 2008, DOI: 10.1186/1754-6834-1-5. [Baidu Scholar]
Li M F, Fan Y M, Xu F, Zhang X L. Cold sodium hydroxide/urea based pretreatment of bamboo for bioethanol production: Characterization of the cellulose-rich fraction. Industrial Crops and Products, 2010, 32(3), 551-559. PBM [Baidu Scholar]